
THE ACCURACY OF AMYVID1
Amyvid has been shown to be accurate for estimating beta-amyloid neuritic plaque density1
Objective evidence of amyloid pathology based on autopsies performed within 1 year of Amyvid scan (n=46)1
- Positive cases (sensitivity): 96% of the time (95% CI: 80% to 100%)1
- Negative cases (specificity): 100% of the time (95% CI: 78% to 100%)1
In a clinical trial, Amyvid identified:1
POSITIVE CASES
(sensitivity)
96%
of the time
(95% CI: 80% to 100%)
NEGATIVE CASES
(specificity)
100%
of the time
(95% CI: 78% to 100%)
STUDY 2 assessed the sensitivity and specificity of a binary visual read methodology in patients who underwent autopsy (truth standard).1
DESIGN1:
- 5 readers (trained in person)
- Binary visual read methodology
POPULATION1:
- 59 terminally ill patients who received premortem Amyvid scans and then underwent autopsy to establish a truth standard
- Of these 59 patients, 29 patients had an Alzheimer’s disease (AD) clinical diagnosis, 13 had another type of dementing disorder, 12 had no history of cognitive impairment, and 5 had MCI
RESULTS:
-
In patients who had an autopsy within 1 year of PET imaging
(n=46)1,2:
- 28 had moderate to frequent plaques and 18 had no or sparse neuritic plaques at autopsy
- The majority of readers rated Amyvid PET scans as positive in 27 of the 28 individuals (1 false positive), giving a sensitivity* of 96% (95% CI: 80% to 100%)
- The majority of readers rated Amyvid PET scans as negative in all 18 individuals (0 false negatives), which corresponded to a specificity* of 100% (95% CI: 78% to 100%)
-
In patients who had an autopsy within 2 years of PET imaging
(n=59)1:
- Sensitivity* was 92% (95% CI: 78% to 98%) and specificity* was 100% (95% CI: 80% to 100%)
*Sensitivity and specificity were calculated using the majority interpretation of the readers for the presence or absence of moderate to frequent amyloid plaques.2
SELECT IMPORTANT SAFETY INFORMATION
Radiation Risk
- Amyvid, similar to other radiopharmaceuticals, contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling to protect patients and health care workers from unintentional radiation exposure
Amyvid is reliable1
Amyvid has been shown to be reliable for estimating beta-amyloid neuritic plaque density1
Statistically significant correlation between Amyvid PET scan images and beta-amyloid plaque deposition seen at autopsy (r=0.78; 95% CI: 0.58 to 0.89; P<0.0001) (n=29).1
NEGATIVE AMYVID
PET SCAN1,3,4†
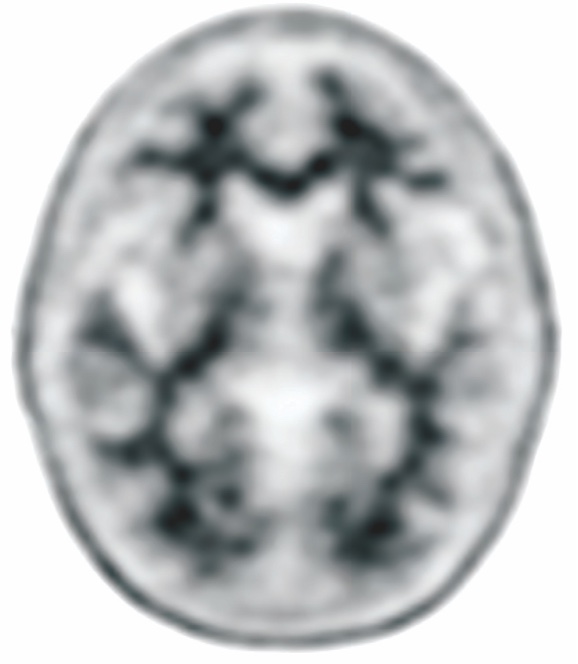
Low Amyvid uptake in cortical gray matter
PATHOLOGY RESULTS (IMMUNOHISTOCHEMISTRY)
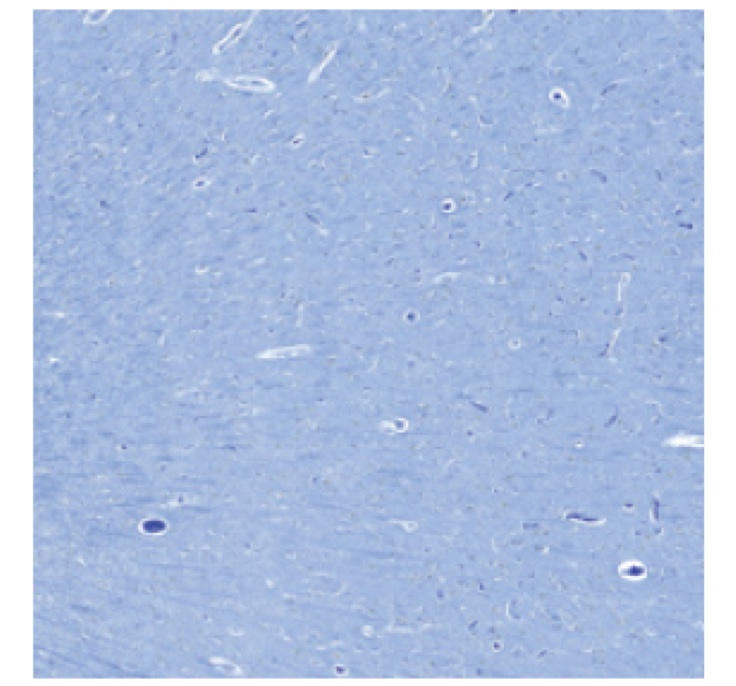
No evidence of amyloid plaques
©2011. Avid Radiopharmaceuticals, Inc. All rights reserved.
†These are examples of a negative amyloid PET scan and pathology slide with no evidence of amyloid plaques.3,4
- Indicates sparse to no amyloid plaques1
- Inconsistent with a neuropathological diagnosis of AD1
- Unlikely that cognitive impairment is due to AD1
- The objective of Amyvid image interpretation is to provide an estimate of the brain beta-amyloid neuritic plaque density, not to make a clinical diagnosis. Image interpretation is performed independently of a patient’s clinical features and relies upon the recognition of unique image features1
POSITIVE AMYVID
PET SCAN1,3,5‡
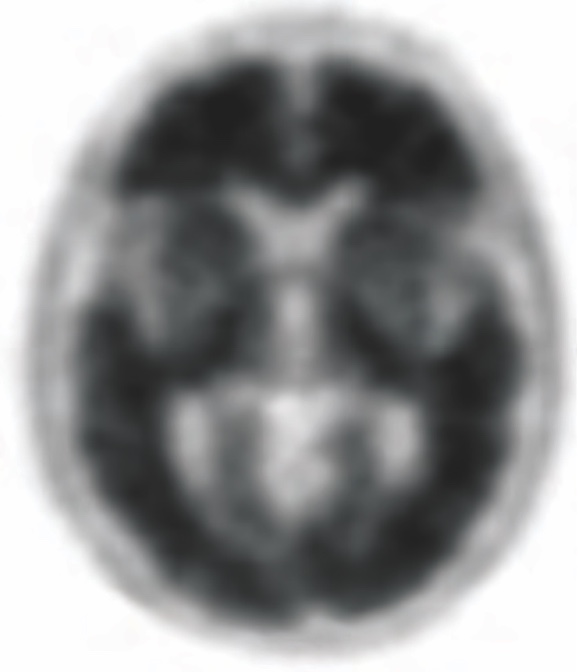
High Amyvid uptake in cortical gray matter
PATHOLOGY RESULTS (IMMUNOHISTOCHEMISTRY)
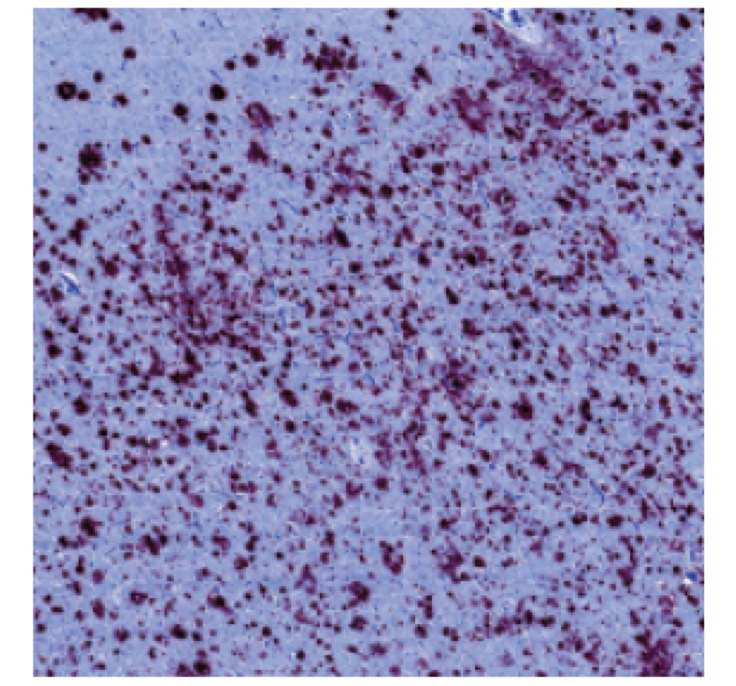
High levels of amyloid plaques
©2011. Avid Radiopharmaceuticals, Inc. All rights reserved.
‡These are examples of a positive amyloid PET scan and pathology slide with high level of amyloid plaques.3,5
- Indicates moderate to frequent amyloid plaques1
- Is consistent with a neuropathological diagnosis of AD1
- This amount of amyloid plaque is seen in patients with AD, but may also be present in patients with other conditions as well as cognitively normal older people1
- A positive Amyvid scan does not establish a diagnosis of AD or other cognitive disorder1
- The objective of Amyvid image interpretation is to provide an estimate of the brain beta-amyloid neuritic plaque density, not to make a clinical diagnosis. Image interpretation is performed independently of a patient’s clinical features and relies upon the recognition of unique image features1
STUDY 1 assessed the correlation between amyloid plaques seen at autopsy and estimates for amyloid plaque density using Amyvid PET imaging.1
DESIGN1:
- 3 readers
- Semiquantitative reading methodology (not intended for clinical use)
POPULATION1:
- 35 terminally ill patients who received premortem Amyvid scans and then underwent autopsy (29 of which comprised the primary analysis set)
SELECT IMPORTANT SAFETY INFORMATION
Risk for Image Misinterpretation and Other Errors
Errors may occur during Amyvid image interpretation. Image interpretation should be performed independently of the patient’s clinical information. Amyvid scan results are indicative of plaque content only at the time of image acquisition and a negative scan does not preclude the development of brain amyloid in the future.
The reproducibility of Amyvid1,6
Amyvid has been shown to have high inter-reader reproducibility for estimating beta-amyloid neuritic plaque density1,6
High inter-reader reproducibility
- 92% of interpretations agreed with the majority (95% CI: 89.8% to 94.1%) (n=59)6
92%
of interpretations agreed with the majority
(95% CI: 89.8% to 94.1%) (n=59)
STUDY 2 assessed the sensitivity and specificity of a binary visual read methodology in patients who underwent autopsy (truth standard).1
DESIGN1:
- 5 readers (trained in person)
- Binary visual read methodology
POPULATION1:
- 59 terminally ill patients|| who received premortem Amyvid scans and then underwent autopsy to establish a truth standard
Study 3: Assessed the inter-and-intra-reader image interpretation reproducibility in subjects with and without autopsy (truth standard)1.
DESIGN1:
- 5 readers (trained using electronic media)
- Binary visual read methodology
POPULATION1:
- 92 subjects¶ who did not undergo autopsy (truth standard) in addition to 59 patients (same population from Study 2) with autopsy data
- 33 randomly selected images were used to assess intra-reader reproducibility
§Fleiss’ kappa: the degree of agreement among
multiple readers over that which would be expected by chance1,2.
||Twenty-nine (29) patients had an AD clinical
diagnosis, 13 had another type of dementing disorder, 12 had no
history of cognitive impairment, and 5 had MCI.1
¶Twenty (20) healthy volunteers, 52 patients with MCI, 20 patients with AD.1
Amyvid clinical studies1
- Amyvid was evaluated in 3 clinical studies that examined images from healthy adult subjects as well as subjects with a range of cognitive disorders, including some terminally ill patients who had agreed to participate in a postmortem brain donation program
- All were single-arm studies in which subjects underwent an Amyvid injection and scan and then had images interpreted by multiple independent readers who were masked to all clinical information
- Image interpretations used co-registration with CT scans when PET scans were performed on dual PET/CT scanners
SELECT IMPORTANT SAFETY INFORMATION
Risk for Image Misinterpretation and Other Errors
The most common adverse reactions reported in clinical trials were headache (1.8%), musculoskeletal pain (0.7%), blood pressure increased (0.7%), nausea (0.7%), fatigue (0.5%), and injection site reaction (0.5%).
Learn more about the Amyvid procedure time
PROCEDURE TIMECI=confidence interval; CT=computed tomography; MCI=mild cognitive impairment; PET=positron emission tomography.
References:
- Amyvid (florbetapir F 18 injection) Prescribing Information. Lilly USA, LLC.
- Clark CM, Pontecorvo MJ, Beach TG, et al; for AV-45-A16 Study Group. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669-678.
- Data on File. Lilly USA, LLC. DOF-AM-US-0006.
- Data on File. Lilly USA, LLC. DOF-AM-US-0008.
- Data on File. Lilly USA, LLC. DOF-AM-US-0007.
- Data on File. Lilly USA, LLC. DOF-AM-US-0005.
